Hebrew University of Jerusalem research unveils “critical insights” into bacterial fruit blotch, which poses a “significant threat” to melons and watermelons
A new study at the Hebrew University of Jerusalem has unveiled insights into bacterial fruit blotch, a severe disease affecting melon and watermelon crops.
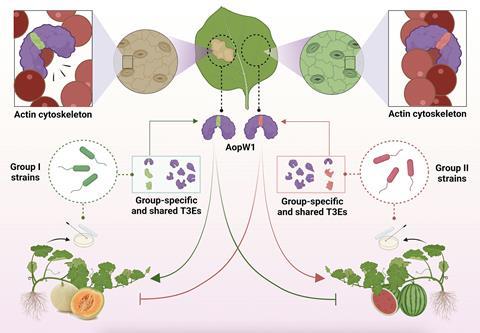
The research focused on the role of the effector AopW1, shedding light on its significance in host adaptation and providing new perspectives on the HopW1 family of bacterial effectors.
Bacterial fruit blotch, caused by the bacterium Acidovorax citrulli, poses a ”significant threat” to melon and watermelon cultivation, the study outlined.
Strains of the pathogen can be classified into two major genetic groups, with group I strains being strongly associated with melon and group II strains having higher aggressiveness towards watermelon.
Previous research at the Hebrew University, led by Professor Saul Burdman, discovered that the two groups of strains differ in the arsenal of type-III secreted protein effectors. These are molecules that are secreted by the bacterium into the host cell where they manipulate its metabolism to promote disease.
On the other hand, certain plant varieties may possess proteins are able to detect the activity of some of these effectors to promote resistance.
The hypothesis is that differences in the arsenal of effectors are major determinants that shape host preferential association in A. citrulli towards melon or watermelon.
Study details
The study focused on one of such effectors, named AopW1. This protein has a highly variable region between amino acids 147 to 192. This region differs in 14 amino acids between strains belonging to the two A. citrulli groups.
Group I’s AopW1 is more harmful to yeast and Nicotiana benthamiana cells, causing stronger disruption to cell structures, increased cell death, and reduced depositions of protective callose as compared to Group II’s AopW1.
The research team demonstrated the significance of specific amino acid positions within this variable region for AopW1’s harmful effects.
AopW1 was also found in different parts of host plant cells, including the endoplasmic reticulum, chloroplasts, and plant endosomes.
Furthermore, the study unveiled a novel aspect of the response to biotic stress by identifying the involvement of the endosome-associated protein EHD1, which, when overexpressed, lessened AopW1-induced cell death and strengthened the plant’s defense mechanisms.
The study showed that inoculation experiments of melon and watermelon using group I and II wild-type and mutant strains, demonstrated that AopW1 not only played a significant role in terms of contribution to virulence of both group I and II strains, but also contributed to shaping host preference of group I and II strains, towards melon and watermelon, respectively.
Deeper understanding
“Our findings provide a deeper understanding of the mechanisms behind bacterial fruit blotch and offer valuable insights into host-pathogen interactions,” said professor Burdman. ”This knowledge is crucial for developing targeted strategies to mitigate the impact of this threatening disease of melon and watermelon crops.”
According to the university, the research not only advances the understanding of plant-pathogenic bacteria, but also opens new avenues for developing innovative approaches to enhance crop resilience against bacterial fruit blotch.
The research paper titled “Natural variation in a short region of the Acidovorax citrulli type III-secreted effector AopW1 is associated with differences in cytotoxicity and host adaptation” is now available in The Plant Journal.



